News Release
RCMG, Inc. is pleased to announce that it has launched a new service for measuring genotype B in your samples as a Contract service [Quantifying HBsAgGi-gB].
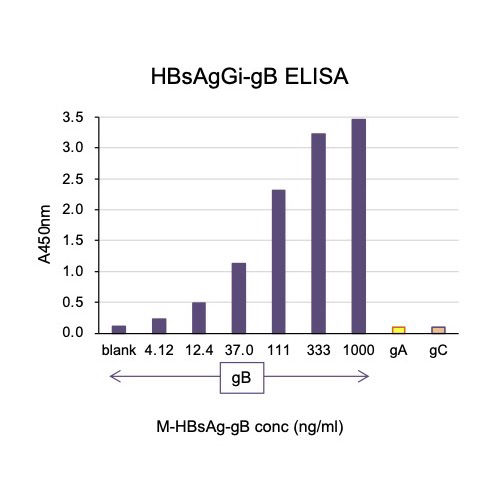
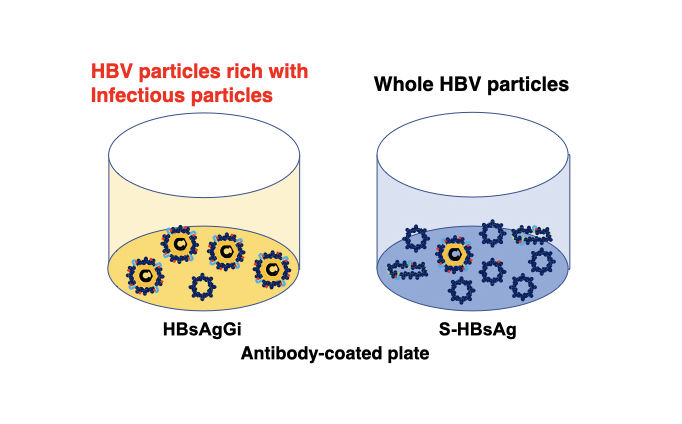
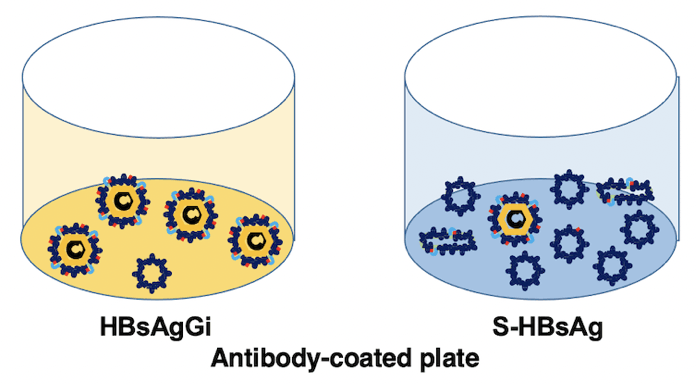
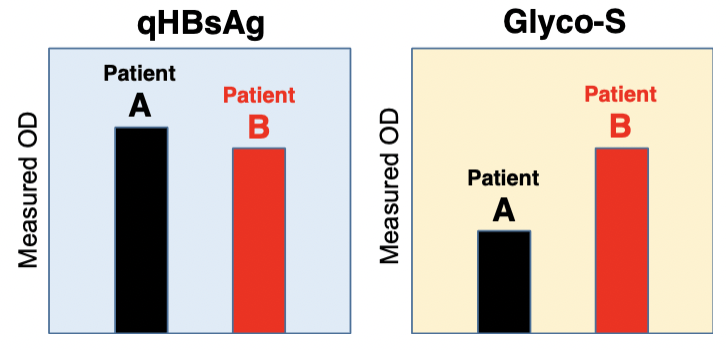
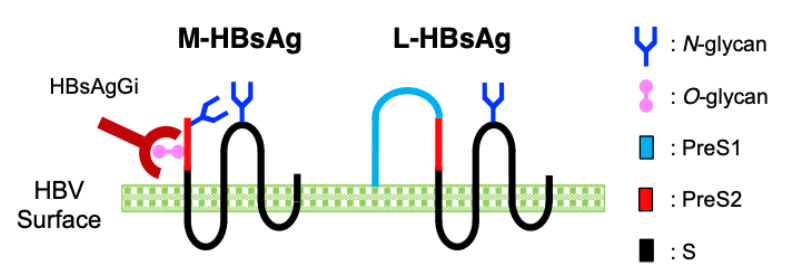
A new antibody, HBsAgGi-gB (HB surface antigen glycan isomer of genotype B), recognizing PreS2 domain of genotype B HBsAg has been established, in addition to HBsAgGi-gC antibody specific to O-glycosylated Pre-S2 on M-HBs. HBV particles captured by HBsAgGi antibodies differ from those captured by S-HBsAg antibody. By using HBsAgG antibodies, we established new ELISA systems to measure HBV DNA- or HBV RNA-containing viral particles of genotype B and C.
By using HBsAgGi ELISA, RCMG will quantify the amount of infectious HBV containing M-HBsAg directly from your clinical or non-clinical samples.
More detail information on this service is available at our PRODUCTS/SERVICES page.